
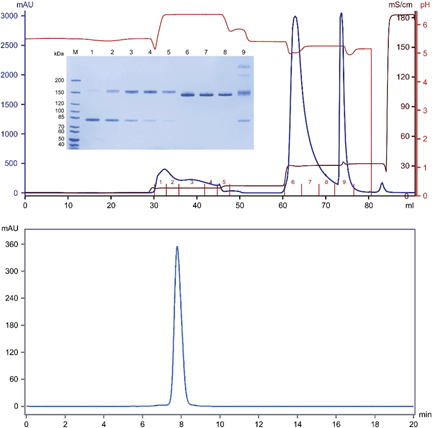
Due to the various fundamental structural similarities between bsAbs and monoclonal antibodies (mAbs), many of the current bsAb downstream purification methodologies are based on the established purification processes of mAbs, where affinity, charge, size, hydrophobicity and mixed-mode-based purification are frequently employed.
The downstream processing of this class of antibodies is therefore of crucial importance in ensuring that these products can be obtained with high purity and yield. Biophys Chem 127:1–8īurgess RR (2018) A brief practical review of size exclusion chromatography: rules of thumb, limitations, and troubleshooting.Bispecific antibodies (bsAbs) represent a highly promising class of biotherapeutic modality. Data file 29-0356-74 ABĪrakawa T, Ejima D, Tsumoto K, Obeyama N, Tanaka Y, Kita Y, Timasheff SN (2007) Suppression of protein interactions by arginine: a proposed mechanism of the arginine effects. GE Healthcare Life Sciences (2015) Capto™ MMC ImpRes. GE Healthcare Life Sciences (2013) Polishing of monoclonal antibodies using Capto™ MMC ImpRes in bind and elute mode.

J Chromatogr A 1294:70–75Ĭhmielowski RA, Meissner S, Roush D, Linden TO, Glowacki E, Konietzko J, Nti-Gyabaah J (2014) Resolution of heterogeneous charged antibody aggregates via multimodal chromatography: a comparison to conventional approaches.

Gao D, Wang LL, Lin DQ, Yao SJ (2013) Evaluating antibody monomer separation from associated aggregates using mixed-mode chromatography. Bioprocess Int 7:52–56Ĭhen J, Tetrault J, Zhang Y, Wasserman A, Conley G, Dileo M, Haimes E, Nixon AE, Ley A (2010) The distinctive separation attributes of mixed-mode resins and their application in monoclonal antibody downstream purification process. Curr Pharm Biotechnol 10:440–446Įriksson K, Ljunglöf A, Rodrigo G, Brekkan E (2009) MAb contaminant removal with a multimodal anion exchanger: a platform step to follow protein A. Gagnon P, Beam K (2009) Antibody aggregate removal by hydroxyapatite chromatography. Gagnon P, Ng P, Aberrin C, Zhen J, He J, Mekosh H, Cummings L, Richieri R, Zaidi S (2006) A ceramic hydroxyapatite based purification platform: simultaneous removal of leached protein A, aggregates, DNA, and endotoxins. McCue JT (2009) Theory and use of hydrophobic interaction chromatography in protein purification applications. Lu Y, Williamson B, Gillespie R (2009) Recent advancement in application of hydrophobic interaction chromatography for aggregate removal in industrial purification process. McCue JT, Engel P, Ng A, Macniven R, Thömmes J (2008) Modeling of protein monomer/aggregate purification and separation using hydrophobic interaction chromatography. Zhang X, Chen T, Li Y (2019) A parallel demonstration of different resins' antibody aggregate removing capability by a case study. He X (2015) Mixed-mode chromatography for mAb S aggregate removal: comparison of CHT™ ceramic hydroxyapatite, capto adhere, and capto adhere impres. Hall T, Wilson JJ, Brownlee TJ, Swartling JR, Langan SE, Lambooy PK (2015) Alkaline cation-exchange chromatography for the reduction of aggregate and a mis-formed disulfide variant in a bispecific antibody purification process. Vázquez-Rey M, Lang DA (2011) Aggregates in monoclonal antibody manufacturing processes. Bonnerjea J, Brake RP, Davis MR, Kellerman K, Preneta A (2008) Ion exchange chromatography and purification of antibodies, United States patent application publication US 2008/0312425 A1


 0 kommentar(er)
0 kommentar(er)
